In a world where the drumbeat for sustainable food solutions is growing louder, a groundbreaking development from the hallowed halls of Tufts University is making waves. The Tufts University Center for Cellular Agriculture (TUCCA), under the stewardship of David Kaplan, Stern Family Professor of Engineering, is redefining the future of meat production. This isn’t just another scientific study; it’s a leap towards a future where your steak doesn’t come from a farm, but a lab.
Revolutionizing Meat Production
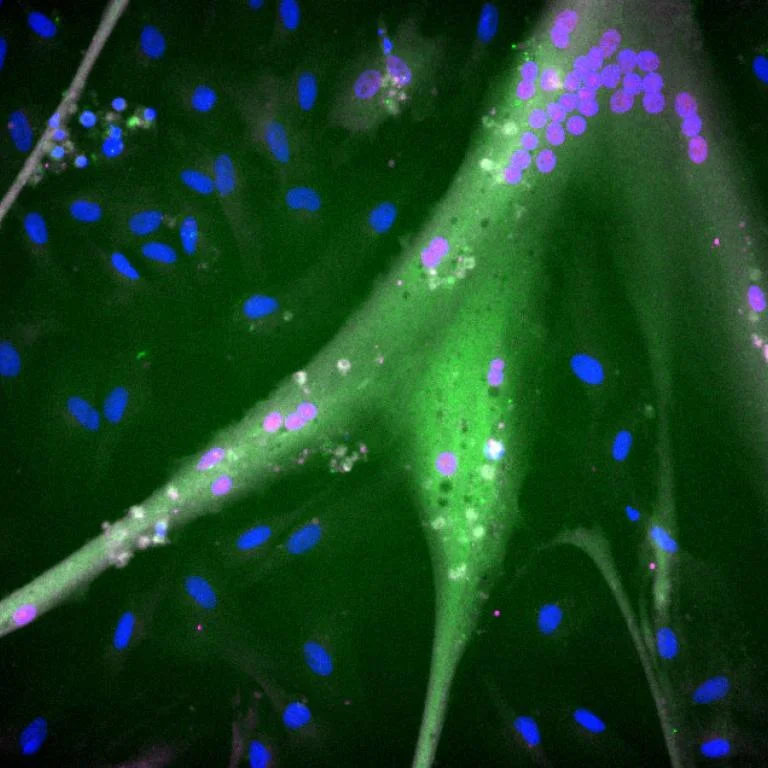
At the heart of this revolution lies a simple, yet transformative idea: creating bovine muscle cells that are not just ordinary cells, but biological factories producing their own growth factors. This innovation could slash the sky-high costs associated with cultivated meat production, making it a viable contender in the food industry.
Imagine a steak that’s not just kind to your wallet, but also to the planet. That’s the promise of this research, published in the journal ‘Cell Reports Sustainability’. By modifying stem cells to produce their own fibroblast growth factor (FGF), the researchers have opened doors to an efficient production of skeletal muscle cells – the kind that ends up as your burger or steak.
Breaking Down the Science
FGF is not exactly a nutrient,” clarifies Andrew Stout, Director of Science at Tufts Cellular Agriculture Commercialization Lab and the project’s lead researcher. “It’s more like an instruction for the cells to behave in a certain way.” By engineering these cells to self-produce growth factors, the team has essentially turned them into self-sustaining units.

Growth factors, a major cost contributor in cultivated meat production, had to be externally added and frequently replenished. Now, by eliminating this requirement, the team has taken a giant leap towards affordability.
Challenges and Optimizations Ahead
While this breakthrough is significant, it’s not without its challenges. Stout acknowledges slower growth in engineered cells but remains optimistic about overcoming this hurdle through various strategies. The focus isn’t just on cost-cutting, but also optimizing for industrial readiness.
This isn’t just about growing meat in a lab. It’s about doing it in a way that eases regulatory pathways. “We’re not adding foreign genes to the cell, just editing and expressing genes that are already there,” Stout explains, highlighting the potential for smoother regulatory approval.
The potential of this technology extends beyond beef to other meats like chicken, pork, or fish. Stout envisions a future where this approach is applied across various meats, tailoring the best growth factors for each species.
The journey to sustainable, lab-grown meat is ongoing, with TUCCA and others continuously working to refine the technology. This includes enhancing the texture, taste, and nutritional content of the meat. Kaplan is optimistic about the future, foreseeing a time when affordable cultivated meat will be a common sight in supermarkets.
More To Discover
- Graveyard of Massive Wind Turbine Blades Have Taken Over Texas Town, Making It the Epicenter of Greenwashed Waste
- Turning Insects Into a Sustainable Solution: The Potential of Bioplastics from Flies
- Netherlands Advances in Renewable Energy with New Solar Bike Paths
- Underwater Kite Generates Sustainable Energy
The path to sustainable meat production is paved with innovation, and Tufts University is leading the charge. In a world grappling with environmental challenges, this breakthrough isn’t just a scientific achievement; it’s a beacon of hope for a more sustainable future.
Source: Cell Reports Sustainability