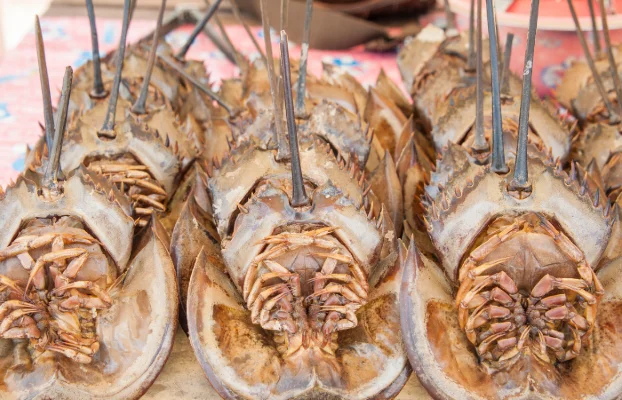
The blood of horseshoe crabs, a species that has roamed our oceans since ancient times, has been an unsung hero in medical safety. However, the tide may be turning with new synthetic alternatives that promise to conserve this unique species.
Horseshoe Crabs: Guardians of Human Health
For those who’ve been vaccinated or received intravenous drugs without subsequent severe fevers, the horseshoe crab has been a silent guardian. Their blood is the source of limulus amebocyte lysate (LAL), a substance that detects harmful bacterial toxins, known as endotoxins, which can cause life-threatening fevers.
The discovery of LAL has been a monumental leap in medical safety, ensuring that intravenous drugs are free from these toxins that can’t be eliminated through sterilization alone.
Yet, this medical marvel comes at a cost to the crab population, stirring debates over the environmental impact and the adoption of synthetic alternatives.
Environmentalists are concerned that a similar fate may await the octopus whose blood doesn’t freeze, considering the scientific possibilities and potential profit from related breakthroughs.
From Ocean Depths to Medical Breakthroughs
The journey to LAL began in the mid-1800s with the advent of intravenous injections. It wasn’t until biochemist Florence Seibert pinpointed water contamination as the cause of “injection fever” that a breakthrough occurred. Her method for detecting and removing fever-inducing substances became a standard by the 1940s.

The rabbit pyrogen test, which involved injecting drugs into rabbits and observing for fever, was the initial method for ensuring drug safety. However, the serendipitous discovery of horseshoe crab blood’s clotting response to endotoxins by Frederik Bang and Jack Levin led to the LAL method. This new approach, which gels in the presence of toxins, became the FDA-approved standard by the 1990s, replacing the rabbit test.
The Harvesting Debate & Impact on Marine Life
The harvesting of horseshoe crabs for LAL production is a complex process with deep environmental and economic implications. Crabs are collected from their natural habitats, primarily along the East Coast of the United States, during their spawning season when they’re most accessible.
Once in laboratories, up to 30% of their unique blue blood is drawn before they’re released back into the ocean. At least that’s how the process is supposed to be handled. In reality, the blood draw is frequently done by unskilled and untrained fishermen right on the boat with the crab tossed back into the water afterward.
Because of this, the precise survival rates post-release are a topic of much debate; estimates vary widely, with some studies suggesting mortality rates as high as 30%.
Financially, the LAL market is lucrative, though exact figures are closely guarded by the industry.
The five main LAL-producing companies operating along the U.S. coastline keep production and sales data proprietary, adding a layer of opacity to the true economic value of the practice. This secrecy also extends to the number of crabs harvested annually, but it’s known that the demand for LAL is high due to its critical role in pharmaceutical testing.
The extraction process isn’t without its critics, who argue that monetary incentives have overshadowed the ecological value of horseshoe crabs. While the crabs themselves aren’t an endangered species, their role in the coastal ecosystem is significant.
Horseshoe crabs do play a critical role in the marine food web, particularly for migratory birds like the red knot, which rely on crab eggs for nourishment. The decline in horseshoe crab numbers due to harvesting for LAL and bait has been linked to the declining populations of these shorebirds, some of which are now threatened.

Synthetic Solutions on the Horizon
The drive to replicate naturally derived medicines in the lab has been ongoing, with notable successes like synthesized insulin. In the 1990s, scientists at the National University of Singapore developed the first synthetic endotoxin detector, recombinant Factor C (rFC), using horseshoe crab genes. Subsequent developments led to recombinant cascade reagents (rCRs), which replicate the entire LAL reaction in the lab.
Despite these advances, LAL remains predominant, partly due to the U.S. Pharmacopeia’s classification of rFC and rCR as “alternative” methods requiring additional validation. The pharmaceutical industry, heavily regulated and accustomed to LAL, has been slow to transition to these synthetics, although some larger companies are starting to make the shift.
The Push for Certification
Conservation groups advocate for the U.S. Pharmacopeia to fully certify rFC, arguing that LAL producers are impeding this to protect their market share. The U.S. Pharmacopeia and LAL producers defend their caution as a necessary measure for public health.
They're Called "Living Fossils" For A Reason

Compare the Jurassic-aged fossil Mesolimulus, found in Solnhofen Limestone in Germany, to horseshoe crabs currently being bled today. They’re identical. Horseshoe crabs have changed very little in 200 million years. walking along the North American coast today. They are practically the same.
The industry’s acknowledgment of recombinant products signifies a shift towards non-horseshoe crab methods. As regulators contemplate new harvest limits and the U.S. Pharmacopeia and FDA review guidance on recombinant alternatives, the public’s voice will play a role in the winter of 2024.
Even without immediate approval for rFC and rCR, more comprehensive data on horseshoe crab populations and greater transparency from the LAL industry would be beneficial steps. Additionally, encouraging medical companies to reserve LAL for final product testing, while utilizing recombinant products during manufacturing, could be a balanced approach.
More To Discover
A Call for Prudent Policy
Creating policies that navigate complex scientific issues is challenging. Incremental steps that safeguard both human health and the environment can lead to significant advances.
As we stand at the crossroads of medical innovation and environmental conservation, the future of one of Earth’s ancient creatures hangs in the balance, prompting a call for careful consideration and action.