In a groundbreaking development, Northwestern University chemists have unveiled a revolutionary catalyst that promises to combat the persistent plastic pollution crisis by breaking down Nylon-6, a highly durable and ubiquitous plastic, within minutes, without generating harmful byproducts.
This game-changing process, recently featured in the journal Chem, not only presents a remarkable solution for environmental remediation but also opens doors to upcycling Nylon-6 waste into higher-value products.
“Plastic is a part of our society; we use so much of it. But the problem is: What do we do when we’re finished with it? Ideally, we wouldn’t burn it or put it into landfills. We would recycle it. We’re developing catalysts that deconstruct these polymers, returning them to their original form so they can be reused,” said Northwestern’s Tobin Marks, the study’s senior author.
Nylon-6 is commonly found in everyday items such as fishing nets, carpets, clothing, and even seat belts. The plastic’s remarkable durability, while advantageous in its intended applications, poses a significant environmental threat when discarded, as it can persist in the environment for thousands of years, contaminating water bodies, causing damage to coral reefs, and endangering various marine species.
“The whole world is aware of the plastic problem,” Marks added.

The issue of Nylon-6 pollution is particularly evident in discarded fishing nets, which account for a substantial portion, roughly 46%, of the Great Pacific Garbage Patch, contributing to an alarming 1 million pounds of fishing gear abandoned in the ocean each year.
These nets, in particular, pose a deadly challenge as they lose their functionality over time, becoming waterlogged and difficult to retrieve, leading to their abandonment in the oceans.

Nylon-6 is commonly found in everyday items such as fishing nets, carpets, clothing, and even seat belts. The plastic’s remarkable durability, while advantageous in its intended applications, poses a significant environmental threat when discarded, as it can persist in the environment for thousands of years, contaminating water bodies, causing damage to coral reefs, and endangering various marine species.
“The whole world is aware of the plastic problem,” Marks added.
“Fishing nets lose quality after a couple of years of use,” said Liwei Ye, the paper’s lead first author who is a postdoctoral fellow in Marks’ laboratory.
Existing methods for disposing of Nylon-6, such as burying it in landfills or burning it, have proven to be ineffective and environmentally damaging. Burning Nylon-6 releases toxic pollutants like nitrogen oxides and carbon dioxide, contributing to health problems and greenhouse gas emissions.

“You can dissolve plastics in acid, but then you are left with dirty water,” Marks said. “What do you do with that? The goal is always to use a green solvent. And what type of solvent is greener than no solvent at all?”
However, the innovative catalyst developed by Northwestern University offers a more sustainable and environmentally friendly solution. Unlike previous catalysts that required extreme conditions, toxic solvents, or high-pressure steam, this catalyst employs yttrium, an abundant and inexpensive metal, and lanthanide ions.
When applied to Nylon-6 samples at melting temperatures without the need for solvents, the plastic rapidly disintegrates, returning to its original building blocks without generating harmful byproducts.
This process recovers an impressive 99% of the plastic’s original monomers, which can then be upcycled into high-value products, such as recycled nylon used by high-end fashion brands.
“You can think of a polymer like a necklace or a string of pearls,” Marks explained. “In this analogy, each pearl is a monomer. These monomers are the building blocks. We devised a way to break down the necklace but recover those pearls.”
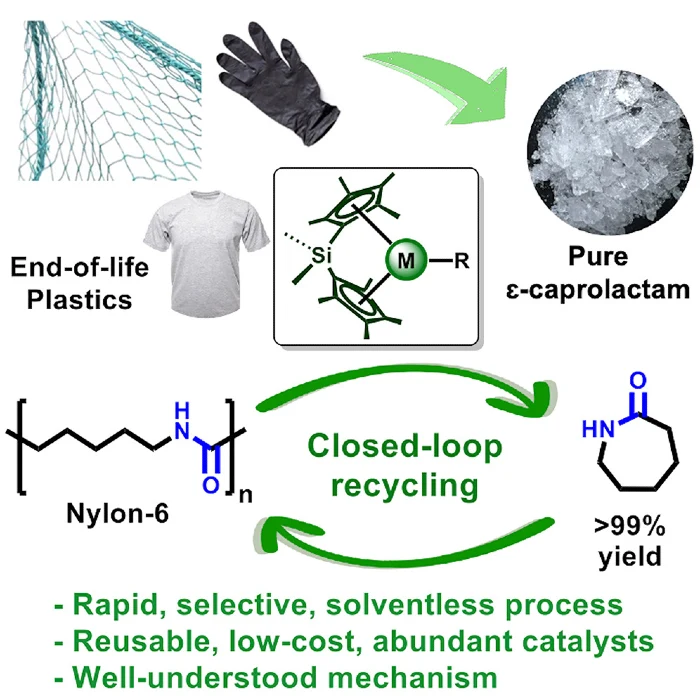
One of the catalyst’s most significant advantages is its high selectivity, specifically targeting Nylon-6 polymers without affecting surrounding materials. This selectivity makes it practical for treating large volumes of unsorted waste, eliminating the need for labor-intensive sorting processes.
“If you don’t have a catalyst that’s selective, then how do you separate the nylon from the rest of the waste?” Marks said. “You would need to hire humans to sort through all the waste to remove the nylon. That’s enormously expensive and inefficient. But if the catalyst only degrades the nylon and leaves everything else behind, that’s incredibly efficient.”
Moreover, by recycling these monomers, there’s a substantial reduction in the need to produce virgin plastics from crude oil, contributing to a more sustainable and eco-friendly approach to plastic production.
“Recycled nylon is actually worth more money than regular nylon,” Marks said. “Many high-end fashion brands use recycled nylon in clothes.”
The researchers have already filed a patent for this groundbreaking process, and interest from potential industrial partners has been robust. This innovation represents a pivotal step forward in the field of polymer recycling and sustainable materials management, offering a practical and efficient solution to the Nylon-6 waste problem.

“Our research represents a significant step forward in the field of polymer recycling and sustainable materials management,” Ye said. “The innovative approach addresses a critical gap in current recycling technologies, offering a practical and efficient solution for the nylon waste problem. We believe it has implications for reducing the environmental footprint of plastics and contributing to a circular economy.”
Funding for this research came from RePLACE (Redesigning Polymers to Leverage A Circular Economy), supported by the U.S. Department of Energy and the National Science Foundation, as well as the Institute for Catalysis in Energy Processes within the Paula M. Trienens Institute for Sustainability and Energy.
More To Discover
- Revolutionizing Glass: A New Type of Glass That Is Greener, Stronger, and Safer
- The Big 57: The Companies Fueling 80% of the World’s Carbon Emissions
- How Chinese Gold Mining is Ravaging Cambodia’s Endangered Wildlife Sanctuary And No One Will Stop Them
- The First Lithium-Free, Sodium Battery EV Will Hit the Roads in January, Proving A Game Changer for the Auto Industry and Environment
This collaborative effort underscores the significance of interdisciplinary research in tackling one of the world’s most pressing environmental challenges.
Source: Chem Journal